RESEARCH ARTICLE
Cost-effectiveness of a multitarget stool DNA test for colorectal cancer screening of Medicare beneficiaries
![]() Steffie K. Naber 1☯*, Amy B. Knudsen2☯, Ann G. Zauber3, Carolyn M. Rutter4, Sara
Steffie K. Naber 1☯*, Amy B. Knudsen2☯, Ann G. Zauber3, Carolyn M. Rutter4, Sara
E. Fischer3¤a, Chester J. Pabiniak5, Brittany Soto3¤b, Karen M. Kuntz6, Iris Lansdorp- Vogelaar1
![]() OPEN ACCESS
OPEN ACCESS
Citation: Naber SK, Knudsen AB, Zauber AG, Rutter CM, Fischer SE, Pabiniak CJ, et al. (2019) Cost-effectiveness of a multitarget stool DNA test for colorectal cancer screening of Medicare beneficiaries. PLoS ONE 14(9): e0220234. https:// doi.org/10.1371/journal.pone.0220234
Editor: Ajay Goel, Beckman Research Institute, UNITED STATES
Received: December 5, 2018
Accepted: July 11, 2019
Published: September 4, 2019
Copyright: © 2019 Naber et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability Statement: All relevant data are within the manuscript and its Supporting Information files.
Funding: This work was supported by contract HHSM-500-2012-00008I with The MITRE
Corporation. The models used in the analysis are supported by the National Institutes of Health under National Cancer Institute Grant U01 CA152959 and U01 CA199335 as part of the Cancer Intervention and Surveillance Modeling Network (CISNET). The authors did not receive any
1 Erasmus MC, University Medical Center Rotterdam, Department of Public Health, Rotterdam, The Netherlands, 2 Institute for Technology Assessment, Massachusetts General Hospital, Boston, Massachusetts, United States of America, 3 Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, New York, United States of America, 4 RAND Corporation, Santa Monica, California, United States of America, 5 Kaiser Permanente Washington Health Research Institute, Seattle, Washington, United States of America, 6 Division of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, Minnesota, United States of America
☯ These authors contributed equally to this work.
¤a Current address: Georgetown University, Washington, D.C., United States of America
¤b Current address: CVS Health, Woonsocket, Rhode Island, United States of America
Abstract
Background
In 2014, the Centers for Medicare and Medicaid Services (CMS) began covering a multitar- get stool DNA (mtSDNA) test for colorectal cancer (CRC) screening of Medicare beneficia- ries. In this study, we evaluated whether mtSDNA testing is a cost-effective alternative to other CRC screening strategies reimbursed by CMS, and if not, under what conditions it could be.
Methods
We use three independently-developed microsimulation models to simulate a cohort of pre- viously unscreened US 65-year-olds who are screened with triennial mtSDNA testing, or one of six other reimbursed screening strategies. Main outcome measures are discounted life-years gained (LYG) and lifetime costs (CMS perspective), threshold reimbursement rates, and threshold adherence rates. Outcomes are expressed as the median and range across models.
Results
Compared to no screening, triennial mtSDNA screening resulted in 82 (range: 79–88) LYG per 1,000 simulated individuals. This was more than for five-yearly sigmoidoscopy (80 (range: 71–89) LYG), but fewer than for every other simulated strategy. At its 2017 reim- bursement rate of $512, mtSDNA was the most costly strategy, and even if adherence were 30% higher than with other strategies, it would not be a cost-effective alternative. At a
other financial support for this work. Model results and the contents of this manuscript are the sole responsibility of the investigators and do not necessarily represent the official views of the National Cancer Institute. The MITRE Corporation and National Institutes of Health had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
substantially reduced reimbursement rate ($6–18), two models found that triennial mtSDNA testing was an efficient and potentially cost-effective screening option.
Conclusions
Compared to no screening, triennial mtSDNA screening reduces CRC incidence and mortal- ity at acceptable costs. However, compared to nearly all other CRC screening strategies reimbursed by CMS it is less effective and considerably more costly, making it an inefficient screening option.
Competing interests: This work was supported by contract HHSM-500-2012-00008I with The MITRE
Corporation. CMR was affiliated with RAND Corporation, but did not receive any financial
support for this work. BS is currently affiliated with
CVS Health but was not affiliated at the time of the study. There are no patents, products in development or marketed products associated with this research to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer death in the United States [1]. Randomized trials of fecal occult blood tests (FOBTs) and flexible sigmoidoscopy have shown that screening can effectively reduce both CRC incidence [2–6] and mortality [2– 12]. In June 2016, the US Preventive Services Task Force updated their CRC screening recom- mendations, including guidelines on the use of FOBTs, flexible sigmoidoscopy, colonoscopy, and the recently-developed multitarget stool DNA test (mtSDNA) [13].
In April 2014, Imperiale et al. [14] published the findings of a study evaluating the test per- formance of the mtSDNA test, Cologuard (Exact Sciences Corporation), based on a single round of screening. Cologuard combines DNA assays for multiple aberrant gene mutations and a proprietary fecal immunochemical assay. Compared with a fecal immunochemical test (FIT), Cologuard demonstrated higher sensitivity for CRC and advanced adenomas but lower specificity. In October 2014, the Centers for Medicare and Medicaid Services (CMS) granted coverage of Cologuard once every 3 years (the interval recommended by the manufacturer) for asymptomatic, average-risk Medicare beneficiaries [15]. Following CMS’s Clinical Laboratory Fee Schedule (CLFS), reimbursement for Cologuard was set by “crosswalking” to comparable diagnostic tests already on the CLFS. This process yielded a 2014 reimbursement rate for Colo- guard of $492.72 per test [16].
CMS requested an analysis of mtSDNA screening of Medicare enrollees from the MITRE Corporation. MITRE commissioned investigators from the Cancer Intervention and Surveil- lance Modeling Network (CISNET) to assess whether mtSDNA testing is a cost-effective alter- native to other CRC screening strategies available to Medicare beneficiaries, and if not, to assess at what reimbursement rate, level of screening uptake, or screening interval it could be a cost-effective option.
Methods
CISNET models
We used three independently-developed microsimulation models of CRC from the National Cancer Institute’s CISNET consortium—the CRC Simulated Population Model for Incidence and Natural History (CRC-SPIN), Microsimulation Screening Analysis for CRC (MISCAN), and Simulation Model of CRC (SimCRC)—to evaluate the cost-effectiveness of screening Medicare beneficiaries with the mtSDNA test, or with an alternative CRC screening approach. All models describe the natural history of CRC in an unscreened population, based on the ade- noma-carcinoma sequence [17–19]. Simulated persons enter free of colonic and rectal lesions at age 20. As they age, they are at risk of developing adenomas. Each adenoma may grow in
size, and some may transition to a preclinical (i.e., undiagnosed) CRC. Preclinical cancers may become symptomatic, at which point the person becomes a clinically-detected case. Persons may die from causes other than CRC at any age, and persons with detected CRC may die from the disease.
Each model has a screening component that allows the natural history of CRC to be inter- rupted due to the detection of a preclinical cancer or the detection and removal of adenoma (s). The chance that screening detects asymptomatic disease in a simulated person depends on the sensitivity of the screening test and, for endoscopic tests, whether the lesion is within the reach of the scope. Screened persons without an underlying lesion may have a false-positive test result and undergo an unnecessary follow-up colonoscopy. Non-adenomatous polyps (e.g., hyperplastic polyps) are not modeled explicitly, but their detection is reflected in the false-positive rates of the tests. The impact of screening depends on the characteristics of the test performed, and on how frequently it is repeated.
CRC screening
We used the models to simulate cohorts of previously unscreened US 65-year-olds, with mtSDNA every 3 years (as specified in the final coverage determination) and six other strate- gies available to Medicare beneficiaries [20]: annual fecal occult blood testing (FOBT) with either a high-sensitivity guaiac-based FOBT (gFOBT) or a FIT, 5-yearly flexible sigmoidos- copy, 10-yearly flexible sigmoidoscopy with annual gFOBT or annual FIT, and 10-yearly colo- noscopy. We assumed all screening begins at age 65 and ends no later than age 75. Individuals with a positive non-colonoscopy screening test undergo a follow-up colonoscopy. Individuals with adenomas detected at a screening or follow-up colonoscopy transition to a surveillance regimen [21], with colonoscopy performed every 3 or 5 years (dependent on findings) until at least age 85.
For the base-case analysis, we assumed 100% adherence to all screening, follow-up, and sur- veillance procedures to assess the effect of screening in those willing to be screened. Alternative assumptions were explored in sensitivity analyses.
Assumptions about test accuracy were based on published findings (Table 1). Complication risks from colonoscopy were obtained from a study by Van Hees et al. [22] that estimated excess risks of serious gastrointestinal events, other gastrointestinal events, and cardiovascular events by age and polypectomy status among Medicare beneficiaries undergoing colonoscopy compared with a matched control group that did not have colonoscopy [23].
Costs
The analysis was conducted from the CMS perspective, and as such, costs were valued by Medicare reimbursement rates and excluded beneficiary copayments and cost-sharing pay- ments. Costs of stool-based screening tests were based on the 2017 Clinical Diagnostic Labora- tory Fee Schedule and costs of endoscopic tests were based on 2014 average Medicare payments (Table 2). For a detailed description of the derivation of these costs, see S1 Appendix. Endoscopy payments were updated to 2017 dollars using the Personal Health Care Deflator Price Index [34]. For each type of colonoscopy complication, the average payment by CMS was calculated using frequency data on hospitalizations for colonoscopy complications (per- sonal communication, Craig Parzynski, MS, of Yale University).
Net costs of CRC-related care by stage at diagnosis and phase of care were obtained from an updated analysis of 2007–2013 SEER-Medicare linked data ([35] and personal communica- tion, Angela Mariotto, PhD) and were updated to 2017 dollars (Table 2).
Table 1. Test characteristics� used in the analysis.
|
|
|
|
Sensitivity Analysis |
||
|
Screening Test Test Characteristic |
Base-Case Value, % |
Source |
Worst-Case Value, % |
Best-Case Value, % |
Source |
|
mtSDNA |
|
Imperiale et al, 2014[14] |
|
|
Imperiale et al, 2014[14] |
|
Specificity† |
89.8 |
|
Not varied |
Not varied |
Not varied |
|
Sensitivity for adenomas <10 mm |
17.2‡ |
|
15.9‡ |
18.6‡ |
|
|
Sensitivity for adenomas �10 mm |
42.4§ |
|
38.7§ |
46.2§ |
|
|
Sensitivity for colorectal cancer |
92.3 |
|
84 |
97 |
|
|
FIT (cutoff 20 μg of hemoglobin per g of feces) |
Imperiale et al, 2014[14] |
|
|
Imperiale et al, 2014[14] |
|
|
Specificity† |
96.4 |
|
Not varied |
Not varied |
Not varied |
|
Sensitivity for adenomas <10 mm |
7.6‡ |
|
6.7‡ |
8.6‡ |
|
|
Sensitivity for adenomas �10 mm |
23.8§ |
|
20.8§ |
27§ |
|
|
Sensitivity for colorectal cancer |
73.8 |
|
62.3 |
83.3 |
|
|
gFOBT |
|
Zauber et al, 2008 [24] |
|
|
|
|
Specificity† |
92.5 |
|
Not varied |
Not varied |
Not varied |
|
Sensitivity for adenomas <6 mm |
7.5k |
|
7.5k |
7.5k |
Zauber et al, 2008[24] |
|
Sensitivity for adenomas 6–9 mm |
12.4 |
|
10 |
26.2 |
Zauber et al, 2008[24] |
|
Sensitivity for adenomas �10 mm |
23.9 |
|
17.7 |
49.4 |
Zauber et al, 2008[24] |
|
Sensitivity for colorectal cancer |
70 |
|
61.5 |
79.4 |
Levi et al, 2011[25] Allison et al, 1996[26] |
|
Colonoscopy (within reach)¶ |
|
|
|
|
|
|
Specificity† |
86�� |
Schroy et al, 2013 [27] |
Not varied |
Not varied |
Not varied |
|
Sensitivity for adenomas <6 mm |
75 |
van Rijn et al, 2006[28] |
70 |
79 |
Zauber et al, 2008[29] |
|
Sensitivity for adenomas 6–9 mm |
85 |
van Rijn et al, 2006[28] |
80 |
92 |
Zauber et al, 2008[29] |
|
Sensitivity for adenomas �10 mm |
95 |
van Rijn et al, 2006[28] |
93.1 |
99.5 |
Johnson et al, 2008[30] |
|
Sensitivity for colorectal cancer |
95 |
By assumption |
93.1 |
99.5 |
By assumption |
|
Reach†† |
95 to end of cecum, remainder between rectum and cecum |
By assumption |
Not varied |
Not varied |
|
|
Sigmoidoscopy (within reach) |
|
|
|
|
|
|
Specificity† |
87�� |
Weissfeld et al, 2005[31] |
Not varied |
Not varied |
|
|
Sensitivity for adenomas <6 mm |
75 |
By assumption |
70 |
79 |
|
|
Sensitivity for adenomas 6–9 mm |
85 |
By assumption |
80 |
92 |
|
|
Sensitivity for adenomas �10 mm |
95 |
By assumption |
93.1 |
99.5 |
|
|
Sensitivity for colorectal cancer |
95 |
By assumption |
93.1 |
99.5 |
|
|
Reach |
76–88 to sigmoid-descending junction; 0 |
Atkin et al, 2002 |
Not varied |
Not varied |
|
|
|
beyond the splenic flexure |
[32] |
|
|
|
|
|
|
Painter et al, 1999 |
|
|
|
|
|
|
[33] |
|
|
FIT = fecal immunochemical test; gFOBT = sensitive guaiac-based fecal occult blood test; mtSDNA = multitarget stool DNA test.
� Sensitivity estimates are per person for stool-based tests and per-lesion for endoscopic tests. Specificity is defined as the probability of a negative test result among persons who do not have adenomas or colorectal cancer.
† Specificity is defined as the probability of a negative test result among persons who do not have adenomas or colorectal cancer.
‡ Sensitivity for persons with non-advanced adenomas. For persons with <6 mm adenomas, we assume that the sensitivity of the test is equal to the positivity rate in persons without adenomas (i.e., 1 –specificity). The sensitivity for persons with 6–9 mm adenomas is chosen such that the weighted average sensitivity for persons with
<6 mm and with 6–9 mm adenoma(s) is equal to that of non-advanced adenomas.
§ Sensitivity for persons with advanced adenomas (i.e., adenomas �10 mm and/or adenomas with advanced histology). Sensitivity was not reported for the subset of
�10 mm adenomas.
k We assume that <6 mm adenomas do not bleed, and therefore cannot cause a positive stool test. We also assume that gFOBT can be positive due to bleeding from other causes, the probability of which is equal to positivity rate in persons without adenomas (i.e., 1–0.925).
¶ We assume the same test characteristics for screening colonoscopies as for colonoscopies for diagnostic follow-up or for surveillance. We assume no correlation in findings between sigmoidoscopy and subsequent diagnostic colonoscopy.
�� The lack of specificity with endoscopy reflects the detection of non-adenomatous polyps, which, in the case of sigmoidoscopy, may lead to unnecessary diagnostic
colonoscopy, and in the case of colonoscopy screening, leads to unnecessary polypectomy, which is associated with an increased risk complications.
†† We assume that 5% of persons undergoing colonoscopy require 2 procedures to achieve complete visualization and that the cecum is ultimately visualized in 95% of patients.
![]()
https://doi.org/10.1371/journal.pone.0220234.t001
Cost-effectiveness analysis
We used the simulation models to calculate lifetime costs of CRC screening and related care and life expectancy for a previously unscreened cohort of 65-year-old Medicare beneficiaries under eight CRC screening strategies, including no screening. We conducted an incremental cost-effectiveness analysis from the perspective of CMS and discounted both future costs and life-years 3% annually to account for time preference for present over future outcomes [36]. Screening strategies were ranked by increasing costs. Strategies that were more costly and less effective than another strategy (i.e., strongly dominated strategies) were eliminated from con- sideration because they were inefficient screening options. Of the remaining strategies, those that were less costly and less effective than another but provided an additional life-year gained (LYG) at a higher incremental cost (i.e., weakly dominated strategies) were also eliminated from consideration. The relative performance of the remaining non-dominated strategies was measured using the incremental cost-effectiveness ratio (ICER), defined as the additional cost of a specific strategy, divided by its additional clinical benefit (in this case, LYG), compared with the non-dominated strategy with costs closest to, but lower than, the strategy of interest.
All non-dominated strategies represent the set of potentially cost-effective options and together comprise the efficient frontier. Which strategy is ultimately deemed to be cost effec- tive depends on the willingness to pay for a LYG. Although there is no official willingness-to- pay (WTP) threshold in the US, a strategy with an ICER less than $50,000–100,000 per LYG is generally considered to provide good value [37]. In this analysis, we assumed a WTP threshold of $100,000 per LYG.
Table 2. Reimbursement for screening tests and for colonoscopy complications, and annual reimbursements for cancer care used in the analysis.
|
Description |
Reimbursement� ($) |
|||
|
Screening tests |
|
|||
|
mtSDNA |
512 |
|||
|
FIT |
22 |
|||
|
gFOBT |
4 |
|||
|
Colonoscopy, without polypectomy, by indication |
|
|||
|
- Screening |
735 |
|||
|
- Diagnostic |
639 |
|||
|
- Surveillance |
724 |
|||
|
Colonoscopy, with polypectomy |
870 |
|||
|
Sigmoidoscopy |
337 |
|||
|
Colonoscopy complications |
|
|||
|
Serious GI complication (perforations, GI bleeding, transfusions) |
6,847 |
|||
|
Other GI complication (paralytic ileus, nausea and vomiting, dehydration, abdominal pain) |
4,878 |
|||
|
Cardiovascular complication (myocardial infarction or angina, arrhythmias, congestive heart failure, cardiac or respiratory arrest, syncope, hypotension, or shock) |
5,347 |
|||
|
Cancer care |
|
|||
|
|
|
Annual reimbursement by stage at diagnosis ($) |
||
|
Phase of care† |
I |
II |
III |
IV |
|
Initial phase |
33,341 |
47,283 |
67,300 |
97,931 |
|
Continuing phase |
2,685 |
3,266 |
5,258 |
26,474 |
|
Terminal phase, non-CRC death |
16,701 |
17,963 |
24,990 |
61,238 |
|
Terminal phase, CRC death |
68,339 |
77,098 |
79,770 |
99,255 |
CRC = colorectal cancer; FIT = fecal immunochemical test; gFOBT = sensitive guaiac-based fecal occult blood test; GI = gastrointestinal; mtSDNA = multitarget stool DNA test.
� All costs are expressed in 2017 US dollars. Costs of stool-based tests are based on the 2017 Centers for Medicare and Medicaid Services Clinical Laboratory Fee
Schedule. Costs of endoscopic procedures are based on 2014 average Medicare payments for a screening sigmoidoscopy and for each type of colonoscopy and include payments for pathology and anesthesia services.
† The initial phase of care is the first 12 months after diagnosis, the last year of life phase is the final 12 months of life, and the continuing phase is all the months between
the initial and last year of life phases.
https://doi.org/10.1371/journal.pone.0220234.t002
Outcomes
Outcomes are reported as the results predicted by each of the three models, focusing on the median prediction, along with estimates from the other two models, which define the range across models.
Sensitivity analyses
Although this analysis was primarily performed to inform reimbursement decisions for Medi- care beneficiaries, we included a sensitivity analysis in which we simulated a 50-year-old cohort.
We also explored the cost-effectiveness of mtSDNA testing using quality-adjusted life years (QALYs) gained as the measure of effectiveness. The assumptions for utilities are provided in S3 Table.
Threshold analyses
If the triennial mtSDNA test strategy was found to be dominated by other screening options, we calculated the maximum cost per mtSDNA test (i.e., the threshold cost) for that strategy to be on the efficient frontier (i.e., to be potentially cost effective). If the mtSDNA test strategy was the least effective strategy, we calculated the threshold cost at which its total discounted cost would be $1 less than the total cost of the least expensive strategy on the efficient frontier
(prior to the addition of the mtSDNA test strategy). If the mtSDNA test strategy was the most effective strategy, we calculated the threshold cost at which its ICER was <$100,000 per LYG. If the mtSDNA test strategy was between the least and most effective strategies on the efficient
frontier, we calculated the threshold cost at which its ICER was equal to the ICER of the effi- cient strategy with LYG closest to and higher than the LYG of the mtSDNA test strategy.
Since the availability of the mtSDNA test could entice a previously unscreened individual to undergo screening, we also identified the threshold mtSDNA test cost for scenarios in which the adherence of the mtSDNA strategy was greater than that of all other screening strategies. For this sensitivity analysis we assumed a fixed overall adherence for each test [38], where those that adhere are completely adherent to screening and those who do not are completely non-adherent. We then increased the relative adherence for the mtSDNA test strategy from 0 to 40% higher than with other tests and calculated the associated threshold mtSDNA test costs. Note that simulating adherence in this manner means that the threshold costs do not change with the overall level of adherence of all other screening strategies, only with the relative increase in adherence with mtSDNA testing.
Threshold costs for mtSDNA were also identified in sensitivity analyses with: higher and lower estimates of the sensitivity of either mtSDNA testing or of all other screening modalities (Table 1); and annual and biennial screening intervals for mtSDNA testing.
Threshold costs are only influenced by the WTP threshold if mtSDNA testing is the most effective strategy. For those threshold analyses, we also present the threshold cost for WTP thresholds of $50,000 and $150,000 per LYG.
Results
In the absence of screening, 64 (range across models: 61–64) per 1,000 65-year-olds will be diagnosed with CRC in their lifetimes (Table 3), resulting in approximately $4.0 million (range: $3.9–4.1 million) in discounted lifetime direct medical costs. All screening strategies yielded large reductions in CRC incidence and mortality. Assuming 100% adherence, the reduction in lifetime risk of CRC with one of the established screening strategies ranged from 50% (range: 36–59%) with annual FIT screening to 73% (range: 58–86%) with 10-yearly colo- noscopy screening (S1 Fig, Panel A). With 46% (range: 33–54%) CRC risk reduction, triennial mtSDNA testing was less effective. Reductions in lifetime risk of CRC death (S1 Fig, Panel B) were higher than reductions in incidence but followed a similar pattern. The reduction in life- time risk of CRC death ranged from 66% (range: 62–68%) with triennial mtSDNA testing to 84% (range: 80–92%) with 10-yearly colonoscopy screening.
Cost-effectiveness analysis
Two screening strategies were found to be efficient by all models: 10-yearly colonoscopy and annual FOBT using either gFOBT or FIT (Fig 1, Table 3). Ten-yearly sigmoidoscopy combined with annual gFOBT was also an efficient screening option in two models (MISCAN and SimCRC).
In one model (MISCAN), triennial mtSDNA testing yielded the fewest LYG of all evaluated strategies, and in the other two models it had the second-fewest LYG after 5-yearly
Table 3. Undiscounted colorectal cancer cases and deaths, and discounted costs and life-years gained with associated incremental cost-effectiveness ratio of no colo- rectal cancer screening and seven colorectal cancer screening strategies in a cohort of 1,000 previously unscreened 65-year-olds, by model.
|
Strategy |
CRC-SPIN |
MISCAN |
SimCRC |
||||||||||||
|
CRC cases |
CRC deaths |
Lifetime costs,� million $ |
LYG� |
ICER, $ |
CRC cases |
CRC deaths |
Lifetime costs,� million $ |
LYG� |
ICER, $ |
CRC cases |
CRC deaths |
Lifetime costs,� million $ |
LYG� |
ICER, $ |
|
|
No screening |
64 |
23 |
3.928 |
0 |
D |
61 |
25 |
3.966 |
0 |
D |
64 |
25 |
4.086 |
0 |
D |
|
gFOBT 1y |
25 |
6 |
2.387 |
89.5 |
- - |
39 |
8 |
3.599 |
86.6 |
D |
31 |
7 |
3.134 |
91.6 |
D |
|
FIT 1y |
27 |
6 |
2.485 |
88.3 |
D |
39 |
8 |
3.561 |
87.2 |
- - |
32 |
7 |
3.131 |
91.9 |
- - |
|
SIG 5y |
29 |
9 |
3.110 |
70.8 |
D |
30 |
7 |
3.878 |
88.9 |
D |
28 |
9 |
3.603 |
80.1 |
D |
|
SIG 10y + gFOBT 1y |
17 |
4 |
2.489 |
99.0 |
D |
29 |
6 |
3.747 |
98.7 |
16,200 |
23 |
5 |
3.282 |
99.1 |
20,900 |
|
SIG 10y + FIT 1y |
17 |
4 |
2.581 |
98.5 |
D |
29 |
6 |
3.782 |
99.0 |
D† |
23 |
5 |
3.320 |
99.3 |
D† |
|
COL 10y |
9 |
2 |
2.479 |
107.4 |
5,100 |
25 |
5 |
3.846 |
101.6 |
34,700 |
17 |
4 |
3.406 |
102.8 |
33,200 |
|
mtSDNA 3y |
30 |
8 |
3.887 |
79.3 |
D |
41 |
9 |
4.889 |
81.7 |
D |
34 |
8 |
4.512 |
87.9 |
D |
- - = default strategy (i.e., the least costly and least effective non-dominated strategy); COL = colonoscopy; CRC = colorectal cancer; D = dominated; FIT = fecal immunochemical test; gFOBT = high sensitivity guaiac-based fecal occult blood test; ICER = incremental cost-effectiveness ratio; LYG = life-years gained compared with no screening; mtSDNA = multitarget stool DNA test; SIG = flexible sigmoidoscopy.
� Future costs and life-years are discounted at a 3% annual rate.
† Indicates a dominated strategy is weakly dominated (i.e., one of the other strategies provides more life-years gained than this strategy, and it has a lower incremental cost-effectiveness ratio). All other dominated strategies are strongly dominated (i.e., provide fewer life-years gained and have higher total costs than another strategy).
![]()
https://doi.org/10.1371/journal.pone.0220234.t003
sigmoidoscopy. Two models (MISCAN and SimCRC) found that triennial mtSDNA testing was the most expensive strategy evaluated; one model (CRC-SPIN) found that only no screen- ing was more expensive than mtSDNA testing. Given the limited LYG and higher costs, trien- nial mtSDNA testing was not an efficient alternative to other strategies reimbursed by CMS.
Sensitivity analyses
Simulating a 50-year-old cohort or using QALYs as the measure of effectiveness did not change the conclusions about the comparative effectiveness and cost-effectiveness of the differ- ent strategies (S4 and S5 Tables, respectively). Triennial mtSDNA testing yielded either the fewest (MISCAN) or second-fewest (CRC-SPIN and SimCRC) LYG; and in two models (MIS- CAN, SimCRC) mtSDNA testing was the most expensive strategy, while in one (CRC-SPIN) only no screening was more costly.
Threshold analyses
In threshold analyses, two models (MISCAN and SimCRC) found that the reimbursement for the mtSDNA test must be considerably lower, in the range of $6–18 per test, for triennial mtSDNA screening to be an efficient and potentially cost-effective strategy (Fig 2). In one model (CRC-SPIN), there was no level of reimbursement at which triennial mtSDNA testing would be cost effective compared with currently recommended screening options (i.e., the threshold cost was negative), due to the benefit being low relative to other tests.
If the triennial mtSDNA test strategy would motivate individuals who would not otherwise be screened to participate in screening, then the threshold cost at which the mtSDNA strategy would be on the efficient frontier would increase. Adherence with mtSDNA testing would need to be 31–53% better than with other tests in order for triennial mtSDNA testing to be effi- cient at the base-case reimbursement rate of $512 (Fig 2, Panel A).
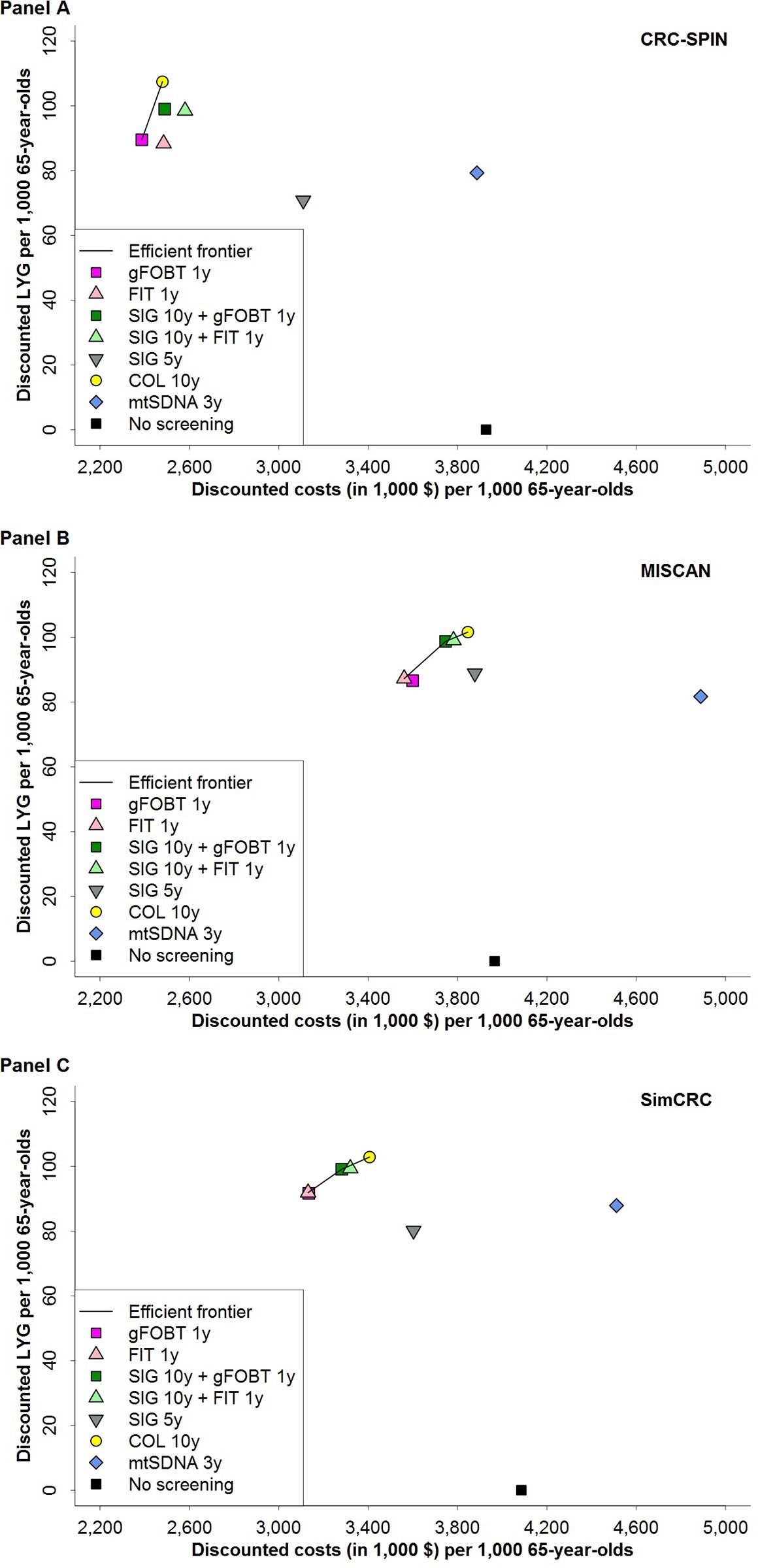
Fig 1. Discounted costs and discounted life-years gained per 1,000 persons aged 65 years for eight colorectal cancer screening strategies and the efficient frontier connecting the economically efficient strategies, for
CRC-SPIN (Panel A), MISCAN (Panel B) and SimCRC (Panel C) models. Discounted costs and life-years gained reflect total costs and life-years gained of a screening program, accounting for time preference for present over future outcomes. Life-years gained are plotted on the y-axis, and total costs are plotted on the x-axis. Each possible screening strategy is represented by a point. Strategies that form the solid line connecting the points lying left and upward are the economically rational subset of choices. This line is called the efficient frontier. The inverse slope of the line represents the incremental cost-effectiveness ratio of the connected strategies. Points lying to the right and beneath the line represent the dominated strategies. Screening with the multitarget stool DNA test every 3 years has higher costs and fewer life-years gained than screening annually with either gFOBT or FIT, and the multitarget stool DNA strategy is therefore strongly dominated. COL = colonoscopy; FIT = fecal immunochemical test; gFOBT = guaiac-based fecal occult blood test; LYG = life-years gained; mtSDNA = multitarget stool DNA test; SIG = flexible sigmoidoscopy.
https://doi.org/10.1371/journal.pone.0220234.g001
If mtSDNA was assumed to perform better at detecting disease or if other modalities were assumed to perform worse than assumed in the base-case analysis, estimated reimbursement rates increased to $32–42 (one model (CRC-SPIN) continued to find no reimbursement rate at which the mtSDNA test strategy would be an efficient option) and $40 (range: $1–52), respectively (S2 Fig). When the interval of mtSDNA screening was shortened to 2 years or 1 year, threshold costs increased to $51 (range: $21–52) and $70 (range: $54–75), respectively (Fig 2, Panel B).
The WTP threshold only influenced the threshold cost of the mtSDNA test when we assumed that adherence with mtSDNA testing would be higher than with other strategies. For SimCRC the threshold cost is affected by the WTP threshold at �17% higher adherence, for MISCAN the threshold cost is affected at �24% higher adherence, and for CRC-SPIN the threshold cost is affected at �36% higher adherence (S3 Fig).
Discussion
This study showed that, with perfect adherence, a 10-year period of triennial mtSDNA screen- ing in previously unscreened 65-year-olds was slightly less effective in terms of LYG than a
10-year period of annual FIT screening. At its 2017 reimbursement rate of $512 per test, trien- nial mtSDNA testing was the most costly strategy and was therefore not cost effective com- pared to other screening options available to Medicare beneficiaries. Per-test reimbursement for the mtSDNA test would need to be less than $6–18 (or even negative, according to one model) for triennial screening to be potentially cost effective compared to other reimbursed strategies. Higher reimbursement rates could be supported with more frequent screening: $51 (range: $21–52) per test with biennial screening and $70 (range: $54–75) per test with annual screening.
These results show that if a previously unscreened 65-year-old were to choose between tri- ennial mtSDNA testing and another reimbursed strategy, it would be both more effective and more cost effective to choose the other strategy. However, mtSDNA testing does reduce CRC incidence and mortality compared to no screening at all. Therefore, mtSDNA testing could be cost effective if it were reserved solely for use by those who are unwilling to participate in CRC screening with any other available test. However, this test has been marketed to the US public for general use, and a study on early adoption of mtSDNA testing found higher usage of mtSDNA among patients with prior CRC screening compared to those without [39].
Despite its higher sensitivity for advanced adenomas and cancer, triennial mtSDNA testing yielded fewer LYG than the two other stool-based CRC screening strategies evaluated. The lower effectiveness can be explained by the longer screening interval. For example, consider a simple case of a person with a single advanced adenoma: if tested with annual FIT, the proba- bility of a positive test is 0.558 after 3 annual screens [i.e., 1—(1–0.238)3], if screening results are independent within an individual. If tested with triennial mtSDNA, there would be only one opportunity for detection in the 3-year period, with a probability of a positive test equal to
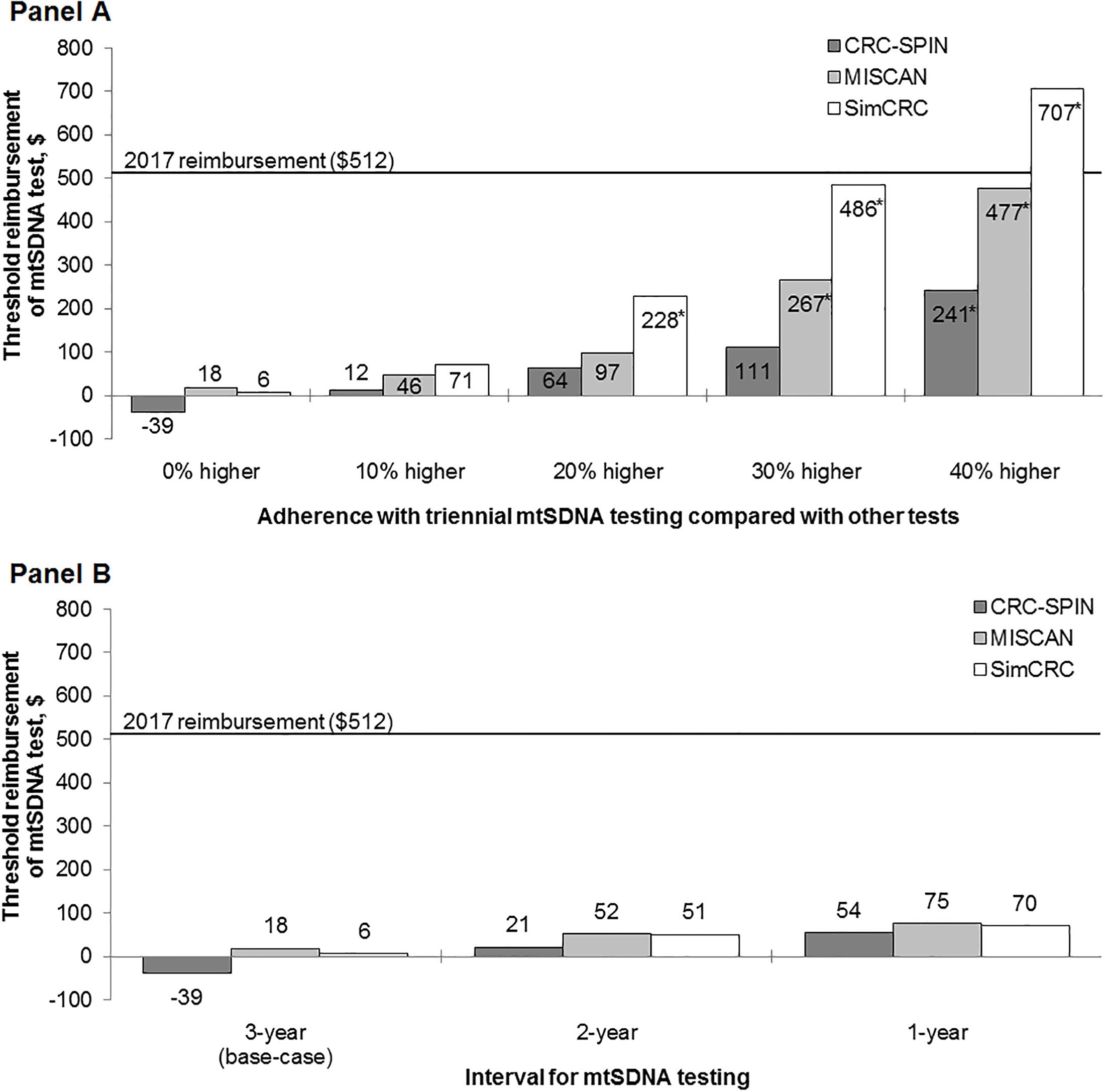
Fig 2. Sensitivity analyses: Reimbursement thresholds for the mtSDNA test at which the mtSDNA test strategy is efficient compared with other reimbursed CRC screening strategies for different levels of adherence with the mtSDNA strategy (Panel A) and for different intervals of screening with the mtSDNA test (Panel B). mtSDNA = multitarget stool DNA test. � For these adherence levels, the threshold cost of mtSDNA test was dependent on the willingness-to-pay threshold. S3 Fig shows the threshold costs for willingness-to-pay thresholds of $50,000 and $150,000 per life year gained.
![]()
https://doi.org/10.1371/journal.pone.0220234.g002
0.424. In summary, if tests are independent within individuals then a program of annual FIT is both more sensitive and more specific than triennial mtSDNA. If performed annually or bien- nially, programmatic sensitivity of mtSDNA screening would increase, but this would likely come at the cost of increased false-positive test results. Therefore, threshold costs only showed a modest increase when the interval was shortened.
When reimbursed at $512 per test, mtSDNA could only be cost effective compared to other strategies if adherence with mtSDNA were considerably higher (31–53% higher) than adher- ence with other tests. The mtSDNA test may appeal to some unscreened persons because it is non-invasive, has higher sensitivity (but lower specificity) than other stool tests and can be per- formed less frequently. However, it is still a stool test (and in fact, requires patients to sample from the collected stool for the immunochemical assay portion of the test), and, as such, the demonstrated barriers to this form of screening, such as handling of stool and storing stool in the house for a short period of time [40] also apply to mtSDNA testing. Furthermore, mtSDNA does not eliminate the barriers common to all screening tests, namely financial barriers, failure of clinicians to advise about CRC screening, and not knowing testing was necessary [40].
CMS’s high reimbursement rate for Cologuard—more than 20 times the reimbursement for other stool-based screening tests for CRC—is the result of federal regulations for setting reimbursement for new diagnostic laboratory tests. Payment for a new diagnostic laboratory test is set by one of two approaches: “cross-walking” or “gap-filling” [41]. Cross-walking is used if the new test is comparable to one or more existing tests already reimbursed under the Clinical Laboratory Fee Schedule (CLFS); reimbursement for the new test is set equal to the reimbursement for the comparable test(s). If no comparable test exists, then payment for a new diagnostic laboratory test is set using the gap-filling approach. With that approach, other information is taken into consideration, including information on reimbursement for the test in non-Medicare settings and resource use required for other relevant tests. Reimbursement for Cologuard was set by cross-walking to three existing codes on the CLFS (81315, 81275, and 82274), yielding the 2014 reimbursement of $492.72 [42]. Had CMS instead used the gap-fill- ing approach, it is possible that CRC screening with Cologuard would have been a cost-effec- tive alternative to other tests reimbursed by CMS. Reimbursement for clinical laboratory tests, including gFOBT, FIT, and Cologuard, changed when Section 216 of the Protecting Access to Medicare Act of 2014 went into effect on January 1, 2018 [43]. In 2019, the reimbursement for gFOBT, FIT, and Cologuard were $4.38, $17.67, and $508.87, respectively.
Our findings are in line with our previous analysis of the cost-effectiveness of stool DNA testing, in which we considered a hypothetical test called “sDNA version 2.0” [44, 45]. This test had sensitivity and specificity similar to the mtSDNA test, but was never available to the public. For that test, we found threshold reimbursement rates of $17–41, which are similar to the cur- rent estimates.
A recent modeling study by Ladabaum and Mannalithara [46] also showed that mtSDNA testing is not cost effective compared to other screening modalities, unless mtSDNA screening resulted in higher participation rates or would cost less than its current reimbursement rate. Our study incorporates three models that all consistently show that adding mtSDNA screening to the menu of CRC screening options results in even lower value than estimated by Ladabaum and Mannalithara. We found that mtSDNA screening is not cost effective even under extremely unrealistic assumptions of a 30% higher adherence than with other tests or test costs of $70. The most likely reason for the difference in threshold costs and adherence, is that Lada- baum and Mannalithara performed their threshold analysis for very specific situations, with either $153 for patient navigation costs added to the cost of FIT, or assuming that only 45% of individuals would ever participate in FIT-based screening.
An important strength of the current study is the use of three independently developed models. Some limitations are noteworthy. First, the models assume that all CRCs arise through the traditional adenoma–carcinoma sequence, and none incorporate a separate pathway for sessile serrated adenomas. However, for both FIT and mtSDNA, the sensitivity for detecting advanced adenomas includes both traditional advanced and sessile serrated adenomas [14].
Our models would underestimate the effectiveness of mtSDNA compared to FIT only if mtSDNA plus diagnostic colonoscopy would detect more serrated adenomas than FIT plus diagnostic colonoscopy and these lesions have higher malignant potential than traditional advanced adenomas. There is evidence to suggest that FIT might be less sensitive than mtSDNA for sessile serrated adenomas [14, 47], but their malignant potential seems compara- ble to that of traditional advanced adenomas [48]. Therefore, the impact on (cost-)effectiveness outcomes of not having two separate pathways to CRC in our models is expected to be limited.
Second, the models simulate the progression from adenoma to CRC by allowing adenomas to increase in size over time. Because adenoma size and presence of villous components or high-grade dysplasia are highly correlated [49], size indirectly represents histology and grade. However, none of the models separately simulate the step from adenoma with low-grade dys- plasia to adenoma with high-grade dysplasia. For the sensitivity of mtSDNA to detect large adenomas (�1 cm), we used the estimate for advanced adenomas from Imperiale et al. [14], who defined advanced adenomas as those with high-grade dysplasia or �25% villous histologic features or measuring �1 cm in size. As colonoscopy sensitivity increases with size, but not histology, of the adenoma, the follow-up colonoscopy after a positive mtSDNA test will detect more high-grade dysplasia and adenomas with villous components if they are all assumed to be of large size, as opposed to if we had modeled histology and grade explicitly. As a result, we may have overestimated the effectiveness of mtSDNA testing.
Third, the simulated cohort did not have any CRC screening prior to age 65. In practice, the Medicare population increasingly consists of individuals who have already had some type of CRC screening. Although the value of screening is lower for individuals with prior screening (because they have lower disease prevalence), the relative difference in effectiveness and cost- effectiveness between different screening strategies is expected to be similar.
Fourth, we assumed conditional independence of repeat screenings. Consequently we assumed that there were no systematic false-negative results for adenomas and cancers. This assumption may not hold for gFOBT and FIT testing because bleeding of a lesion may not be a random event [50]. However, this assumption may not hold for mtSDNA testing either, because testing for blood is an important component of the test. Furthermore, the lesion in question may have acquired a gene mutation not assessed by the mtSDNA test, which means our assumption of conditional independence may be less likely to hold for the mtSDNA test compared to gFOBT and FIT. As a result, we may have overestimated the benefit of stool tests in general and of mtSDNA testing in particular, and its threshold reimbursement rate may be even lower than estimated here.
Finally, because test-specific data on longitudinal screening patterns are lacking, our base- case analysis assumes 100% adherence with screening, follow-up and surveillance procedures. Uptake of screening among the Medicare population is considerably less than 100% [38], as is adherence with repeat screening [51], follow-up [52] and surveillance [53]. Meanwhile, over- use of resources is also common [54], and a positive stool test is sometimes followed by another stool test instead of by the prescribed follow-up colonoscopy [55]. Although we did include a sensitivity analysis with lower uptake, we assumed that individuals were either fully adherent or fully non-adherent with screening. The impact of less-than-perfect adherence among those who take up screening will vary according to the interval of testing and the char- acteristics of the test. While stool-based testing may have higher initial acceptance rates,[56–
59] patients’ willingness to comply with annual stool-based testing over longer periods of time is uncertain,[60–62] and no data is available on adherence with triennial mtSDNA testing.
In summary, our analysis shows that compared with no screening, triennial mtSDNA test- ing reduces CRC incidence and mortality. However, with perfect adherence, it is less effective than other CRC screening options available to Medicare beneficiaries. At its current reim- bursement rate, triennial mtSDNA testing also has higher costs than all other strategies, mak- ing it an inefficient screening option. It could be efficient and potentially cost effective if mtSDNA testing would increase adherence with CRC screening to nearly 100%. Triennial (or more frequent) mtSDNA testing could also be potentially cost effective if the reimbursement rate were substantially lower, i.e. similar to that of other stool-based tests.
Supporting information
S1 Fig. Reductions in the lifetime risk of being diagnosed with (Panel A) and dying from (Panel B) colorectal cancer. Median reduction and the range across models are shown for each screening strategy. COL = colonoscopy; CRC = colorectal cancer; FIT = fecal immuno- chemical test; gFOBT = high sensitivity guaiac-based fecal occult blood test;
mtSDNA = multitarget stool DNA test; SIG = flexible sigmoidoscopy. (TIF)
S2 Fig. Sensitivity analyses: Reimbursement thresholds for the mtSDNA test at which the mtSDNA test strategy is efficient compared with other reimbursed CRC screening strate- gies for different levels of the sensitivity of the mtSDNA test (Panel A), and for different levels of the sensitivity of all other tests (Panel B). mtSDNA = multitarget stool DNA test. � See Table 1 for the worst-case, base-case, and best-case test sensitivities for mtSDNA and all other tests.
(TIF)
S3 Fig. Sensitivity analyses: Reimbursement thresholds for the mtSDNA test at which the mtSDNA test strategy is efficient compared with other reimbursed CRC screening strate- gies for different levels of adherence with the mtSDNA strategy relative to other strategies, assuming a willingness-to-pay threshold of $50,000 per life-year gained (Panel A) and
$150,000 per life-year gained (Panel B). mtSDNA = multitarget stool DNA test. (TIF)
S1 Table. Current Procedural Terminology codes and Healthcare Common Procedure Coding System codes used for colorectal cancer screening, follow-up, and surveillance tests and procedures, and mean reimbursement for the test/procedure and accompanying pathology and anesthesia services [1–3]. COL = colonoscopy; CPT code = Current Proce- dural Terminology code; FIT = fecal immunochemical test; gFOBT = guaiac-based fecal occult blood test; HCPCS code = Healthcare Common Procedure Coding System code;
mtSDNA = multitarget stool DNA test. � Includes facility payments, when appropriate. † Reimbursement for pathology services was assumed to apply only to colonoscopy procedures in which polypectomy was performed. ‡ Sigmoidoscopy is simulated without biopsy or poly- pectomy of detected lesions. § If multiple polyps are removed by different methods (S2 Table), more than one CPT code may be submitted for one colonoscopy. In such cases, payment for all but the highest-reimbursed procedure is reduced to the difference between the payment for the procedure of interest and the payment for basic washing of the colon (CPT 45378). (DOCX)
S2 Table. Descriptions of the surgical Current Procedural Terminology codes used for colonoscopies with biopsy or polypectomy. CPT code = Current Procedural Terminology code. � Code was deleted in 2015 and replaced with 45388.
(DOCX)
S3 Table. Utility decrements used in the sensitivity analysis with quality-adjusted life years as the measure of effectiveness. These decrements were applied to age-based utility weights for general health [1]. CRC = colorectal cancer; FIT = fecal immunochemical test;
gFOBT = sensitive guaiac-based fecal occult blood test; GI = gastrointestinal; LY = life year; mtSDNA = multitarget stool DNA test. � Disutility of colonoscopy (0.12) is from Swan et al. [2]. Duration of disutility (1.5 days, or 0.41% of a year) is from Jonas et al. [3]. † Disutility of sigmoidoscopy was assumed to be the same as for colonoscopy. Duration of disutility was assumed to be 0.4 days, 0.12% of a year. ‡ Disutility of complications was assumed to be 0.5. Duration of disutility was assumed to be 4 days, 2 days, and 3.5 days for serious GI, other GI, and cardiovascular complications respectively. § The initial phase of care is the first 12 months after diagnosis, the last year of life phase is the final 12 months of life, and the continuing phase is all the months between the initial and last year of life phases. Disutilities per LY with CRC were derived from Ness et al. [4].
(DOCX)
S4 Table. Undiscounted colorectal cancer cases and deaths, and discounted costs and life- years gained with associated incremental cost-effectiveness ratio of no colorectal cancer screening and seven colorectal cancer screening strategies in a cohort of 1,000 previously unscreened 50-year-olds, by model. — = default strategy (i.e., the least costly and least effec- tive non-dominated strategy); COL = colonoscopy; CRC = colorectal cancer; D = dominated; FIT = fecal immunochemical test; gFOBT = high sensitivity guaiac-based fecal occult blood test; ICER = incremental cost-effectiveness ratio; LYG = life-years gained compared with no screening; mtSDNA = multitarget stool DNA test; SIG = flexible sigmoidoscopy. � Future costs and life-years are discounted at a 3% annual rate. † Indicates a dominated strategy is weakly dominated (i.e., one of the other strategies provides more life-years gained than this strategy, and it has a lower incremental cost-effectiveness ratio). All other dominated strategies are strongly dominated (i.e., provide fewer life-years gained and have higher total costs than another strategy).
(DOCX)
S5 Table. Discounted costs and quality-adjusted life-years gained with associated incre- mental cost-effectiveness ratio of no colorectal cancer screening and seven colorectal can- cer screening strategies in a cohort of 1,000 previously unscreened 65-year-olds, by model.
— = default strategy (i.e., the least costly and least effective non-dominated strategy);
COL = colonoscopy; CRC = colorectal cancer; D = dominated; FIT = fecal immunochemical test; gFOBT = high sensitivity guaiac-based fecal occult blood test; ICER = incremental cost- effectiveness ratio; QALYG = quality-adjusted life-years gained compared with no screening; mtSDNA = multitarget stool DNA test; SIG = flexible sigmoidoscopy. � Future costs and qual- ity-adjusted life-years are discounted at a 3% annual rate. † Indicates a dominated strategy is weakly dominated (i.e., one of the other strategies provides more quality-adjusted life-years gained than this strategy, and it has a lower incremental cost-effectiveness ratio). All other dominated strategies are strongly dominated (i.e., provide fewer quality-adjusted life-years gained and have higher total costs than another strategy).
(DOCX)
S1 Appendix. Calculation of screening costs.
(DOCX)
Acknowledgments
We thank Joel V. Brill, MD, of Predictive Health, LLC for his assistance with coding of colorec- tal cancer screening procedures; Janet Brock, Linda Gousis, JD, and Stephanie Bartee of the Centers for Medicare and Medicaid Services for providing data on reimbursement for colorec- tal cancer screening procedures; Craig Parzynski, MS, of Yale University and Martha Morton of Reimbursement Principles for assistance with estimation of the reimbursement for treat- ment of colonoscopy complications; Angela Mariotto, PhD, of the National Cancer Institute (NCI), for providing estimates of the cost of cancer care; and Eric “Rocky” Feuer, PhD, for continued support of the CISNET Colorectal Cancer Working Group. We also thank Juan Arroyo, PhD, for project management support.
Personal communication from Craig Parzynski regarding analysis of colonoscopy frequen- cies was for work he conducted under contract HHSM-500-2013-13018I, Task Order HHSM- 500-T0002, Measure & Instrument Development and Support (MIDS): Development, Reeval- uation, and Implementation of Outpatient Outcome/Efficiency Measures, funded by the Cen- ters for Medicare & Medicaid Services, an agency of the US Department of Health and Human Services.
Author Contributions
Conceptualization: Steffie K. Naber, Amy B. Knudsen, Ann G. Zauber, Carolyn M. Rutter, Karen M. Kuntz, Iris Lansdorp-Vogelaar.
Formal analysis: Steffie K. Naber, Amy B. Knudsen, Carolyn M. Rutter, Chester J. Pabiniak, Karen M. Kuntz, Iris Lansdorp-Vogelaar.
Funding acquisition: Ann G. Zauber.
Investigation: Steffie K. Naber, Amy B. Knudsen, Carolyn M. Rutter, Sara E. Fischer, Chester
J. Pabiniak, Brittany Soto, Karen M. Kuntz, Iris Lansdorp-Vogelaar.
Project administration: Sara E. Fischer, Brittany Soto. Resources: Ann G. Zauber, Sara E. Fischer, Brittany Soto. Supervision: Ann G. Zauber, Iris Lansdorp-Vogelaar.
Visualization: Amy B. Knudsen.
Writing – original draft: Steffie K. Naber, Amy B. Knudsen, Iris Lansdorp-Vogelaar.
Writing – review & editing: Ann G. Zauber, Carolyn M. Rutter, Sara E. Fischer, Chester J. Pabiniak, Brittany Soto, Karen M. Kuntz.
References
1. American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society; 2017.
2. Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al. The effect of fecal occult- blood screening on the incidence of colorectal cancer. N Engl J Med. 2000; 343(22):1603–7. https://doi. org/10.1056/NEJM200011303432203 PMID: 11096167.
3. Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sig- moidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lan- cet. 2010; 375(9726):1624–33. Epub 2010/05/01. https://doi.org/10.1016/S0140-6736(10)60551-X PMID: 20430429.
4. Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, et al. Once-only sigmoidoscopy in colo- rectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial—SCORE. J Natl Cancer Inst. 2011; 103(17):1310–22. Epub 2011/08/20. https://doi.org/10.1093/jnci/djr284
5. Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, et al. Colorectal-cancer inci- dence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012; 366(25):2345–57. Epub 2012/05/23. https://doi.org/10.1056/NEJMoa1114635 PMID: 22612596.
6. Holme O, Loberg M, Kalager M, Bretthauer M, Hernan MA, Aas E, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014; 312 (6):606–15. Epub 2014/08/15. https://doi.org/10.1001/jama.2014.8266 PMID: 25117129.
7. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999; 91(5):434–7. https://doi.org/10.1093/jnci/91. 5.434 PMID: 10070942.
8. Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013; 369(12):1106–14. Epub 2013/09/21. https://doi. org/10.1056/NEJMoa1300720 PMID: 24047060.
9. Scholefield JH, Moss SM, Mangham CM, Whynes DK, Hardcastle JD. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut. 2012; 61(7):1036–40. Epub 2011/11/05. https://doi.org/10.1136/gutjnl-2011-300774 PMID: 22052062.
10. Kronborg O, Jorgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol. 2004; 39(9):846– 51. https://doi.org/10.1080/00365520410003182 PMID: 15513382.
11. Lindholm E, Brevinge H, Haglind E. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. Br J Surg. 2008; 95(8):1029–36. https://doi.org/10.1002/bjs.6136 PMID: 18563785.
12. Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, et al. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004; 126 (7):1674–80. Epub 2004/06/10. https://doi.org/10.1053/j.gastro.2004.02.018 PMID: 15188160.
13. U. S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr., et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommen- dation Statement. JAMA. 2016; 315(23):2564–75. Epub 2016/06/16. https://doi.org/10.1001/jama. 2016.5989 PMID: 27304597.
14. Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014; 370(14):1287–97. Epub 2014/03/22. https://doi.org/10.1056/NEJMoa1311194 PMID: 24645800.
15. Centers for Medicare and Medicaid Services. Decision Memo for Screening for Colorectal Cancer— Stool DNA Testing (CAG-00440N) 2014 [updated October 9, 2014December 9, 2015]. https://www. cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=277.
16. Centers for Medicare and Medicaid Services. 2014 Clinical Laboratory Fee Schedule (CLAB) Public Use File (PUF). www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/ clinlab.html or www.cms.gov/apps/ama/license.asp?file=/ClinicalLabFeeSched/downloads/14CLAB. zip. 2014.
17. Morson B. President’s address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974; 67(6 Pt 1):451–7. Epub 1974/06/01. PMID: 4853754.
18. Morson BC. Evolution of cancer of the colon and rectum. Cancer. 1974; 34(3):suppl:845–9. PMID: 4851945.
19. Morson BC. The evolution of colorectal carcinoma. Clin Radiol. 1984; 35(6):425–31. Epub 1984/11/01. https://doi.org/10.1016/s0009-9260(84)80033-1 PMID: 6499378.
20. American Cancer Society. Colorectal Cancer Screening: Insurance Coverage: American Cancer Soci- ety; 2016 [updated June 24th, 2016; cited 2016 July 19th]. www.cancer.gov.
21. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012; 143(3):844–57. Epub 2012/07/06. https://doi.org/10. 1053/j.gastro.2012.06.001 PMID: 22763141.
22. van Hees F, Zauber AG, Klabunde CN, Goede SL, Lansdorp-Vogelaar I, van Ballegooijen M. The appropriateness of more intensive colonoscopy screening than recommended in Medicare beneficia- ries: a modeling study. JAMA Intern Med. 2014; 174(10):1568–76. Epub 2014/08/19. https://doi.org/10. 1001/jamainternmed.2014.3889 PMID: 25133641.
23. Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, et al. Adverse events after out- patient colonoscopy in the Medicare population. Ann Intern Med. 2009; 150(12):849–57, W152. https:// doi.org/10.7326/0003-4819-150-12-200906160-00008 PMID: 19528563.
24. Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008; 149(9):659–69. Epub 2008/10/08. https://doi.org/10.7326/0003- 4819-149-9-200811040-00244 PMID: 18838717.
25. Levi Z, Birkenfeld S, Vilkin A, Bar-Chana M, Lifshitz I, Chared M, et al. A higher detection rate for colo- rectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasi- bility study. Int J Cancer. 2011; 128(10):2415–24. Epub 2010/07/27. https://doi.org/10.1002/ijc.25574 PMID: 20658527.
26. Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal- cancer screening. N Engl J Med. 1996; 334(3):155–9. Epub 1996/01/18. https://doi.org/10.1056/ NEJM199601183340304 PMID: 8531970.
27. Schroy PC 3rd, Coe A, Chen CA, O’Brien MJ, Heeren TC. Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med. 2013; 159(1):13–20. Epub 2013/07/03. https://doi.org/10.7326/0003-4819-159-1-201307020-00004 PMID: 23817700.
28. van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate deter- mined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006; 101(2):343–50. Epub 2006/02/04. https://doi.org/10.1111/j.1572-0241.2006.00390.x PMID: 16454841.
29. Zauber AG, Levin TR, Jaffe CC, Galen BA, Ransohoff DF, Brown ML. Implications of new colorectal cancer screening technologies for primary care practice. Med Care. 2008; 46(9 Suppl 1):S138–46. Epub 2008/09/09. https://doi.org/10.1097/MLR.0b013e31818192ef PMID: 18725826.
30. Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, et al. Accuracy of CT colono- graphy for detection of large adenomas and cancers. N Engl J Med. 2008; 359(12):1207–17. Epub 2008/09/19. https://doi.org/10.1056/NEJMoa0800996 PMID: 18799557.
31. Weissfeld JL, Schoen RE, Pinsky PF, Bresalier RS, Church T, Yurgalevitch S, et al. Flexible sigmoidos- copy in the PLCO cancer screening trial: results from the baseline screening examination of a random- ized trial. J Natl Cancer Inst. 2005; 97(13):989–97. Epub 2005/07/07. https://doi.org/10.1093/jnci/dji175 PMID: 15998952.
32. Atkin WS, Cook CF, Cuzick J, Edwards R, Northover JM, Wardle J, et al. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet. 2002; 359(9314):1291–300. Epub 2002/04/20. https://doi.org/10.1016/S0140-6736(02)08268-5 PMID: 11965274.
33. Painter J, Saunders DB, Bell GD, Williams CB, Pitt R, Bladen J. Depth of insertion at flexible sigmoidos- copy: implications for colorectal cancer screening and instrument design. Endoscopy. 1999; 31(3): 227–31. Epub 1999/05/27. https://doi.org/10.1055/s-1999-13673 PMID: 10344426.
34. National Health Expenditure Data. Table 23. National Health Expenditures; Nominal Dollars, Real Dol- lars, Price Indexes, and Annual Percent Change: Selected Calendar Years 1980–2017 [Internet]. 2017 [cited February 4, 2019]. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics- Trends-and-Reports/NationalHealthExpendData/Downloads/Tables.zip.
35. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011; 103(2):117–28. Epub 2011/01/14. https://doi.org/ 10.1093/jnci/djq495
36. Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost- effectiveness in Health and Medicine. JAMA. 1996; 276(15):1253–8. PMID: 8849754.
37. Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008; 8(2):165–78. Epub 2008/04/01. https://doi.org/10. 1586/14737167.8.2.165 PMID: 20528406.
38. Sabatino SA, White MC, Thompson TD, Klabunde CN. Cancer screening test use—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015; 64(17):464–8. Epub 2015/05/08.
39. Rutten LJF, Jacobson RM, Wilson PM, Jacobson DJ, Fan C, Kisiel JB, et al. Early Adoption of a Multi- target Stool DNA Test for Colorectal Cancer Screening. Mayo Clin Proc. 2017; 92(5):726–33. https:// doi.org/10.1016/j.mayocp.2017.01.019 PMID: 28473037
40. Jones RM, Woolf SH, Cunningham TD, Johnson RE, Krist AH, Rothemich SF, et al. The relative impor- tance of patient-reported barriers to colorectal cancer screening. Am J Prev Med. 2010; 38(5):499–507. Epub 2010/03/30. https://doi.org/10.1016/j.amepre.2010.01.020 PMID: 20347555.
41. Code of Federal Regulations (CFR) 414.508—Payment for a new clinical diagnostic laboratory test, (2011).
42. Centers for Medicare and Medicaid Services (CMS). Clinical Laboratory Fee Schedule (CLFS): Final Determinations. 2015.
43. Protecting Access to Medicare Act of 2014, (2014).
44. Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, van Ballegooijen M, Kuntz KM. Cost-Effec- tiveness of DNA Stool Testing to Screen for Colorectal Cancer: Report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MISCAN and SimCRC Models. 2007. www.cms.hhs.gov/mcd/viewtechassess.asp?from2=viewtechassess.asp&id=212&.
45. Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, Wilschut JA, Zauber AG, van Ballegooijen M. Stool DNA testing to screen for colorectal cancer in the Medicare population: a cost-effectiveness analysis. Ann Intern Med. 2010; 153(6):368–77. Epub 2010/09/22. https://doi.org/10.7326/0003-4819-153-6- 201009210-00004 PMID: 20855801.
46. Ladabaum U, Mannalithara A. Comparative Effectiveness and Cost-Effectiveness of a Multi-target Stool DNA Test to Screen for Colorectal Neoplasia. Gastroenterology. 2016. Epub 2016/06/18. https:// doi.org/10.1053/j.gastro.2016.06.003
47. Heigh RI, Yab TC, Taylor WR, Hussain FT, Smyrk TC, Mahoney DW, et al. Detection of colorectal ser- rated polyps by stool DNA testing: comparison with fecal immunochemical testing for occult blood (FIT). PLoS One. 2014; 9(1):e85659. Epub 2014/01/28. https://doi.org/10.1371/journal.pone.0085659 PMID: 24465639.
48. Holme O, Bretthauer M, Eide TJ, Loberg EM, Grzyb K, Loberg M, et al. Long-term risk of colorectal can- cer in individuals with serrated polyps. Gut. 2015; 64(6):929–36. Epub 2014/11/18. https://doi.org/10. 1136/gutjnl-2014-307793 PMID: 25399542.
49. O’Brien MJ, Winawer SJ, Zauber AG, Gottlieb LS, Sternberg SS, Diaz B, et al. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990; 98(2):371–9. PMID: 2403953.
50. van der Meulen MP, Lansdorp-Vogelaar I, van Heijningen EM, Kuipers EJ, van Ballegooijen M. Non- bleeding adenomas: Evidence of systematic false-negative fecal immunochemical test results and their implications for screening effectiveness-A modeling study. Cancer. 2016; 122(11):1680–8. Epub 2016/ 04/12. https://doi.org/10.1002/cncr.29952 PMID: 27061710.
51. Gellad ZF, Stechuchak KM, Fisher DA, Olsen MK, McDuffie JR, Ostbye T, et al. Longitudinal adherence to fecal occult blood testing impacts colorectal cancer screening quality. Am J Gastroenterol. 2011; 106 (6):1125–34. Epub 2011/02/10. https://doi.org/10.1038/ajg.2011.11 PMID: 21304501.
52. Partin MR, Burgess DJ, Burgess JF, Jr, Gravely A, Haggstrom D, Lillie SE, et al. Organizational predic- tors of colonoscopy follow-up for positive fecal occult blood test results: an observational study. Cancer Epidemiol Biomarkers Prev. 2015; 24(2):422–34. Epub 2014/12/05. https://doi.org/10.1158/1055-9965. EPI-14-1170 PMID: 25471345.
53. Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Reding DJ, Hayes RB, et al. Utilization of surveil- lance colonoscopy in community practice. Gastroenterology. 2010; 138(1):73–81. Epub 2009/10/13. https://doi.org/10.1053/j.gastro.2009.09.062 PMID: 19818779.
54. Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010; 152(10):668–76. Epub 2010/04/15. https://doi.org/10.7326/0003-4819-152-10-201005180-00239 PMID: 20388703.
55. Miglioretti DL, Rutter CM, Bradford SC, Zauber AG, Kessler LG, Feuer EJ, et al. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Med Care. 2008; 46(9 Suppl 1):S91–6. Epub 2008/09/09. https://doi.org/10.1097/MLR.0b013e31817946c8 PMID: 18725839.
56. Gupta S, Halm EA, Rockey DC, Hammons M, Koch M, Carter E, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal can- cer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013; 173 (18):1725–32. Epub 2013/08/08. https://doi.org/10.1001/jamainternmed.2013.9294 PMID: 23921906.
57. Hol L, van Leerdam ME, van Ballegooijen M, van Vuuren AJ, van Dekken H, Reijerink JC, et al. Screen- ing for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut. 2010; 59(1):62–8. Epub 2009/08/13. https://doi.org/10. 1136/gut.2009.177089 PMID: 19671542.
58. Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012; 172(7):575–82. Epub 2012/04/12. https://doi.org/10.1001/archinternmed.2012.332 PMID: 22493463.
59. Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas A, et al. Colonoscopy versus fecal immu- nochemical testing in colorectal-cancer screening. N Engl J Med. 2012; 366(8):697–706. Epub 2012/ 02/24. https://doi.org/10.1056/NEJMoa1108895 PMID: 22356323.
60. Kapidzic A, Grobbee EJ, Hol L, van Roon AH, van Vuuren AJ, Spijker W, et al. Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol. 2014; 109(8):1257–64. Epub 2014/07/02. https://doi.org/10.1038/ajg.2014.168 PMID: 24980879.
61. Jensen CD, Corley DA, Quinn VP, Doubeni CA, Zauber AG, Lee JK, et al. Fecal Immunochemical Test Program Performance Over 4 Rounds of Annual Screening: A Retrospective Cohort Study. Ann Intern Med. 2016; 164(7):456–63. Epub 2016/01/27. https://doi.org/10.7326/M15-0983 PMID: 26811150.
62. Crotta S, Segnan N, Paganin S, Dagnes B, Rosset R, Senore C. High rate of advanced adenoma detec- tion in 4 rounds of colorectal cancer screening with the fecal immunochemical test. Clin Gastroenterol Hepatol. 2012; 10(6):633–8. Epub 2012/03/20. https://doi.org/10.1016/j.cgh.2012.02.030 PM
ID: 22426085.

